Dysmorphology
Introduction
The Dysmorphology, Bone and Cartilage Screen of the GMC contributes the phenotyping for bone and cartilage related phenotypes as well as for morphological abnormalities. The aim of the screen is to establish mouse models for human skeletal diseases like osteoporosis, scoliosis, limb defects, osteogenesis imperfecta or osteoarthritis.
Primary Screen
For the primary screen, we cover a broad spectrum of parameters of bone development, bone metabolism and homeostasis. We have implemented an experimental set up, which enables us to perform high throughput non-invasive first-line phenotyping for bone and cartilage abnormalities. In addition, we developed a protocol (54 parameters) for a quick anatomical observation of animals, which is able to detect and evaluate malformations and malfunctions of the different organ systems.
Analysis
1. Dysmorphological Analysis:
Quick and easy whole body check up for anatomical abnormalities and malfunctions in different organ systems.
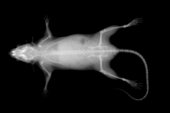

2. X-ray Analysis:
Within a few seconds the whole mouse skeleton can be visualised, X-rays of special parts can be obtained in higher magnification, enabling us to:
- Judge the appearance and presence of all bones and joints.
- Gather information about developmental disorders and, to a minor extent, bone metabolic disorders.
3. Bone densitometry:
With dual-energy X-ray absorptiometry (DXA), the mouse skeleton can be analysed allowing:
- Rapid quantitative measurements of bone mineral density and body composition.
- Study of bone mineralisation and homeostasis defects (e.g., osteoporosis, osteogenesis imperfecta).
Secondary Screen
In secondary tests, we evaluate mutants with altered parameters in the primary screen as model systems for human bone related diseases like scoliosis, limb defects, osteoporosis, osteogenesis imperfecta or osteoarthritis by more detailed phenotypic characterisation.
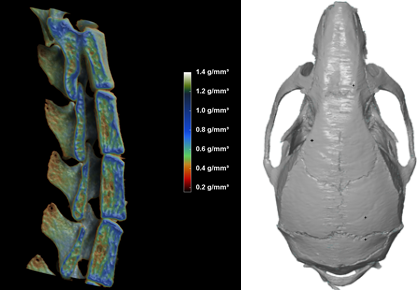
1. Micro computed tomography (µCT):
- Provides a detailed view of the abnormality (in vivo imaging with a resolution down to 9 microns).
- Enables volumetric bone density measurements in g/cm³ of the appendicular skeleton and tail vertebra. Separate analysis of cortical and trabecular bone compartments (BMD, BMC, bone area).
2. Markers of bone formation and resorption:
- Upon request, analysis of bone formation and resorption markers in serum/plasma by ELISA can be performed.
3. Mechanical bending: three-point bend test (long term availability):
- Measurement of the elastic-plastic bone material properties and fracture parameters.
4. Skeleton preparation:
- Staining with alcian blue (cartilage) and alizarin red (bone)
References (selected publications)
Swan AL, Schütt C, Rozman J, Del Mar Muñiz Moreno M, Brandmaier S, Simon M, Leuchtenberger S, Griffiths M, Brommage R, Keskivali-Bond P, Grallert H, Werner T, Teperino R, Becker L, Miller G, Moshiri A, Seavitt JR, Cissell DD, Meehan TF, Acar EF, Lelliott CJ, Flenniken AM, Champy MF, Sorg T, Ayadi A, Braun RE, Cater H, Dickinson ME, Flicek P, Gallegos J, Ghirardello EJ, Heaney JD, Jacquot S, Lally C, Logan JG, Teboul L, Mason J, Spielmann N, McKerlie C, Murray SA, Nutter LMJ, Odfalk KF, Parkinson H, Prochazka J, Reynolds CL, Selloum M, Spoutil F, Svenson KL, Vales TS, Wells SE, White JK, Sedlacek R, Wurst W, Lloyd KCK, Croucher PI, Fuchs H, Williams GR, Bassett JHD, Gailus-Durner V, Herault Y, Mallon AM, Brown SDM, Mayer-Kuckuk P, Hrabe de Angelis M; IMPC Consortium. Mouse mutant phenotyping at scale reveals novel genes controlling bone mineral density. PLoS Genet. 2020 Dec 28;16(12):e1009190
Ignatova VV, Stolz P, Kaiser S, Gustafsson TH, Lastres PR, Sanz-Moreno A, Cho YL, Amarie OV, Aguilar-Pimentel A, Klein-Rodewald T, Calzada-Wack J, Becker L, Marschall S, Kraiger M, Garrett L, Seisenberger C, Hölter SM, Borland K, Van De Logt E, Jansen PWTC, Baltissen MP, Valenta M, Vermeulen M, Wurst W, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Rando OJ, Kellner SM, Bultmann S, Schneider R. The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 2020 May 1;34(9-10):715-729
Abe K, Cox A, Takamatsu N, Velez G, Laxer RM, Tse SML, Mahajan VB, Bassuk AG, Fuchs H, Ferguson PJ, Hrabe de Angelis M. Gain-of-function mutations in a member of the Src family kinases cause autoinflammatory bone disease in mice and humans. Proc Natl Acad Sci U S A. 2019 Jun 11;116(24):11872-11877
Fuchs H, Sabrautzki S, Przemeck GK, Leuchtenberger S, Lorenz-Depiereux B, Becker L, Rathkolb B, Horsch M, Garrett L, Östereicher MA, Hans W, Abe K, Sagawa N, Rozman J, Vargas-Panesso IL, Sandholzer M, Lisse TS, Adler T, Aguilar-Pimentel JA, Calzada-Wack J, Ehrhard N, Elvert R, Gau C, Hölter SM, Micklich K, Moreth K, Prehn C, Puk O, Racz I, Stoeger C, Vernaleken A, Michel D, Diener S, Wieland T, Adamski J, Bekeredjian R, Busch DH, Favor J, Graw J, Klingenspor M, Lengger C, Maier H, Neff F, Ollert M, Stoeger T, Yildirim AÖ, Strom TM, Zimmer A, Wolf E, Wurst W, Klopstock T, Beckers J, Gailus-Durner V, Hrabé de Angelis M. The First Scube3 Mutant Mouse Line with Pleiotropic Phenotypic Alterations. G3 (Bethesda). 2016 Dec 7;6(12):4035-4046.
Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A, Krause M, Koehne T, Albers J, Schulze J, Schilling S, Haberland M, Denninger H, Neven M, Hermans-Borgmeyer I, Streichert T, Breer S, Barvencik F, Levkau B, Rathkolb B, Wolf E, Calzada-Wack J, Neff F, Gailus-Durner V, Fuchs H, de Angelis MH, Klutmann S, Tsourdi E, Hofbauer LC, Kleuser B, Chun J, Schinke T, Amling M. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat Commun. 2014 Oct 21;5:5215
Fuchs H, Gau C, Hans W, Gailus-Durner V, Hrabě de Angelis M. Long-term experiment to study the development, interaction, and influencing factors of DEXA parameters. Mamm Genome. 2013 Oct;24(9-10):376-88
Hochrath K, Ehnert S, Ackert-Bicknell CL, Lau Y, Schmid A, Krawczyk M, Hengstler JG, Dunn J, Hiththetiya K, Rathkolb B, Micklich K, Hans W, Fuchs H, Gailus-Durner V, Wolf E, de Angelis MH, Dooley S, Paigen B, Wildemann B, Lammert F, Nüssler AK. Modeling hepatic osteodystrophy in Abcb4 deficient mice. Bone. 2013 Aug;55(2):501-11
Thiele F, Cohrs CM, Przemeck GK, Wurst W, Fuchs H, Hrabé de Angelis M. In vitro analysis of bone phenotypes in Col1a1 and Jagged1 mutant mice using a standardized osteoblast cell culture system. J Bone Miner Metab. 2013 May;31(3):293-303
Fuchs H, Sabrautzki S, Seedorf H, Rathkolb B, Rozman J, Hans W, Schneider R, Klaften M, Hölter SM, Becker L, Klempt M, Elvert R, Wurst W, Klopstock T, Klingenspor M, Wolf E, Gailus-Durner V, de Angelis MH. Does enamelin have pleiotropic effects on organs other than the teeth? Lessons from a phenotyping screen of two enamelin-mutant mouse lines. Eur J Oral Sci. 2012 Aug;120(4):269-77
Fuchs H, Gailus-Durner V, Adler T, Aguilar-Pimentel JA, Becker L, Calzada-Wack J, Da Silva-Buttkus P, Neff F, Götz A, Hans W, Hölter SM, Horsch M, Kastenmüller G, Kemter E, Lengger C, Maier H, Matloka M, Möller G, Naton B, Prehn C, Puk O, Rácz I, Rathkolb B, Römisch-Margl W, Rozman J, Wang-Sattler R, Schrewe A, Stöger C, Tost M, Adamski J, Aigner B, Beckers J, Behrendt H, Busch DH, Esposito I, Graw J, Illig T, Ivandic B, Klingenspor M, Klopstock T, Kremmer E, Mempel M, Neschen S, Ollert M, Schulz H, Suhre K, Wolf E, Wurst W, Zimmer A, Hrabě de Angelis M. Mouse phenotyping. Methods. 2011; Feb;53(2):120-135.
Abe K, Fuchs H, Boersma A, Hans W, Yu P, Kalaydjiev S, Klaften M, Adler T, Calzada-Wack J, Mossbrugger I, Rathkolb B, Rozman J, Prehn C, Maraslioglu M, Kametani Y, Shimada S, Adamski J, Busch DH, Esposito I, Klingenspor M, Wolf E, Wurst W, Gailus-Durner V, Katan M, Marschall S, Soewarto D, Wagner S, de Angelis MH. A novel ENU-induced mutation in Phospholipase C gamma 2 causes inflammatory arthritis, metabolic defects, and in vitro male infertility in the mouse. Arthritis Rheum.-US. 2011; 63 (5): 1301–1311.
Lisse TS, Thiele F, Fuchs H, Hans W, Przemeck GK, Abe K, Rathkolb B, Quintanilla-Martinez L, Hoelzlwimmer G, Helfrich M, Wolf E, Ralston SH, Hrabé de Angelis M. ER Stress-Mediated Apoptosis in a New Mouse Model of Osteogenesis imperfecta. PLoS Genet. 2008 Feb;4(2):e7
Fuchs, H., Lisse, T., Hans, W., Abe, K., Thiele, F., Gailus-Durner, V., Hrabé de Angelis, M. Phenotypic characterization of mouse models for bone-related diseases in the German Mouse Clinic. J. Musculoskelet. Neuronal. Interact. 2008 Jan-Mar;8(1):13-4.
Fuchs H, Lisse T, Abe K, Hrabé de Angelis M. Screening for bone and cartilage phenotypes in mice. In: Standards of Mouse Model Phenotyping. Eds.: Hrabé de Angelis M., Chambon P. and Browns S. Wiley-VCH Verlag GmbH, Weinheim (Germany). 2006; pp. 35-86.
Fuchs H, Schughart K, Wolf E, Balling R, Hrabé de Angelis M. Screening for dysmorphological abnormalities - a powerful tool to isolate new mouse mutants. Mamm Genome. 2000 Jul;11(7):528-30.

